Our research group uses an integrated structural biology, computational and biophysical approach combined with functional studies to understand the inhibition mechanisms of novel protein-RNA targets involved in human diseases and translate it to develop novel therapeutics.
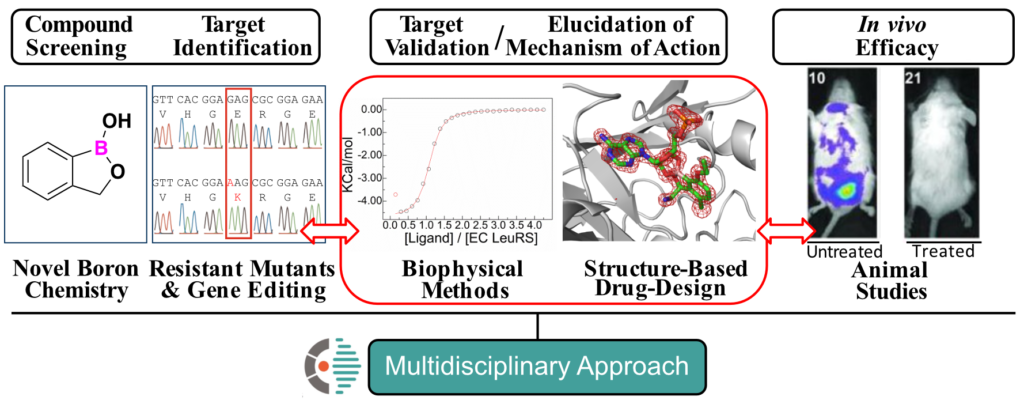
We are interested in elucidating the molecular basis of the inhibition mechanism of novel drug targets. In collaboration with other groups in academia and industry, we are improving the potency and selectivity of inhibitors with biomedical applications.
Selected Publications
– Targeting a microbiota Wolbachian aminoacyl-tRNA synthetase to block its pathogenic host. Science Adv. 10(28) 10.1126/sciadv.ado1453 (2024).
– Hoffmann, G., Le Gorrec, M., Mestdach, E., Cusack, S., Salmon, L., *Jensen, MR. & *Palencia, A. Adenosine-Dependent Activation Mechanism of Prodrugs Targeting an Aminoacyl-tRNA Synthetase. Journal of the American Chemical Society. 145(2), 800-810 (2023).
– Mariño Perez, L., Ielasi, F.S., Bessa, L.M., Maurin, D., Kragelj, J., Blackledge, M., Salvi, N., Bouvignies, G., *Palencia, A., *Jensen, M.R. Visualizing protein breathing motions associated with aromatic ring flipping. Nature 602, 695-700 (2022).
– Ielasi, FS., Ternifi, S., Fontaine, E., Iuso, D., Couté, Y. & A. Palencia. Human Histone pre-mRNA Assembles Histone- or Canonical-mRNA Processing Complexes by Overlapping 3’-End Sequence Elements. Nucleic Acids Research. 50 (21), 12425-12443 (2022).
– Lukarska, M. & *Palencia, A. Aminoacyl-tRNA synthetases as drug targets. The Enzymes, Elsevier. 48:321-350. doi: 10.1016/bs.enz.2020.07.001. (2020).
– Swale, C., Bougdour, A., Gnahoui-David, A., Tottey, J., Georgeault, S., *Laurent, F., *†Palencia, A. *†Hakimi, MA. Metal-captured inhibition of pre-mRNA processing activity by CPSF3 controls Cryptosporidium infection. Science Trans. Medicine 6;11(517). doi: 10.1126 (2019). †Lead Author.
– *Palencia, A., *Bougdour, A., et al. Targeting Toxoplasma gondii CPSF3 as a new approach to control toxoplasmosis. EMBO Molecular Medicine. 9: 385-394 (2017). Highlighted in: France 3 news.
– *Palencia, A., Li, X., et al. Discovery of Novel Protein Synthesis Inhibitors of M.tuberculosis That Target Leucyl-tRNA Synthetase. Antimicrobial Agents & Chemotherapy. 60(10):5817-27 (2016).
– Chopra, S.†, Palencia, A.†, et al. Structural characterisation of antibiotic self-immunity tRNA synthetase in plant tumour biocontrol agent. Nature comm 7:12928 (2016).
– Palencia, A.†,Chopra, S.†, et al. Plant tumour biocontrol agent employs a tRNA-dependent mechanism to inhibit leucyl-tRNA synthetase. Nature comm 4, 1417 (2013).
– Palencia, A., Crepin, T., et al. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nature Struct. & Mol. Biol. 19, 677-84 (2012).
Palencia’s Group, Institute for Advanced Biosciences (IAB), Inserm U1209, CNRS UMR5309/ Université Grenoble-Alpes, Grenoble.
Contact: andres.palencia [ at] univ-grenoble-alpes.fr